Potential Solutions
The ocean, which covers more than 70% of our planet, may be able to help. In a few key parts of the ocean, biological activity is limited by a lack of iron in seawater. Adding iron could, therefore, help spur growth of phytoplankton and increase both the uptake of carbon dioxide by the ocean and the amount that gets sequestered at depth.
Science needs to lead the way, by providing the greatest possible insight into both the intended and unintended consequences, as well as the long-term effectiveness of adding iron to the ocean.
The decisions we make today will shape the future of generations to come.
Iron Fertilization FAQ
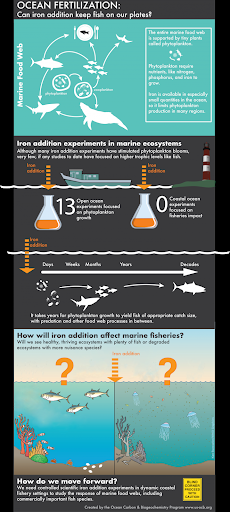
All organisms require nutrients to live and grow, and those living in the open ocean are no exception. Tiny phytoplankton drift on the water’s surface, converting sunlight, water, and carbon dioxide from the atmosphere into food and oxygen. Like plants on land, these tiny algae and cyanobacteria require nutrients for photosynthesis to occur. On land, nitrogen and phosphorus are often the limiting nutrients, but in many parts of the open ocean, it’s other trace nutrients, particularly iron, that are missing.
As a result, adding small amounts of iron to the ocean’s surface can stimulate massive blooms of phytoplankton. Such additions happen naturally, such as when winds blow dust from the Sahara Desert or ash from a volcanic eruption onto the ocean surface. This material provides the iron needed to stimulate phytoplankton growth and removes substantial amounts of carbon dioxide from the atmosphere. Iron fertilization is a Carbon Dioxide Removal (CDR) strategy that would mimic this natural system by artificially adding iron to the ocean surface to stimulate growth of phytoplankton.